Greetings, science enthusiasts and curious minds alike! Have you ever marveled at the incredible efficiency of your body, especially the way it transports and uses oxygen? We’re diving into a less commonly known but utterly fascinating aspect of physiology – the Haldane Effect. This intriguing phenomenon is crucial in how our blood carries carbon dioxide away from our tissues and helps us breathe more easily.
The Haldane effect is the opposite of the Bohr effect. The Bohr impact tells us that the concentration of carbon dioxide and hydrogen ions in the blood affects hemoglobin’s affinity for Oxygen. It increases the amount of oxygen that the exercising tissue can absorb. Also, it increases the amount of oxygen the blood can absorb from our lungs’ alveoli.
What does the Haldane effect do? It talks about how oxygen concentration inside the red blood cells inside our blood affects hemoglobin’s affinity for carbon dioxide and Hydrogen ions. Also, It increases the mouth of Carbon dioxide that the exercising tissue can release into the blood. It increases the number of Carbon dioxide molecules the lungs’ alveoli can absorb from the blood plasma.
Take a deep breath, and let’s go on an insightful journey to understand the Haldane Effect, uncovering the science behind how our bodies master the art of gas exchange. Ready to be amazed by the wonders of your physiology? Let’s get started!
What Is Haldane Effect?
Haldane effect favors carbon dioxide unloading or loading by the change in the pressure of oxygen. It means oxygen pressure changes help to load or unload carbon dioxide. In the lungs, oxygen pressure is high. So, it helps unload the carbon dioxide to be released. In the tissues, oxygen pressure is low, allowing the carbon dioxide to be picked up. So, that is called the Haldane effect.
Tissue makes carbon dioxide that comes out. The carbon dioxide comes in, and it loads. Most carbon dioxide becomes the HCO3 ion and moves bicarb form to the lungs. It is the reverse of the Bohr effect.
Reaction: CO2 + H2O <=> H+ + HCO3 –
Haldane Effect Physiology
The Haldane Effect is a physiological phenomenon that describes the increased capacity of deoxygenated blood to carry carbon dioxide (CO2) and, conversely, the reduced capacity of oxygenated blood to carry CO2. Named after the British scientist J.B.S. Haldane, who first described this effect in the early 20th century, it plays a crucial role in the efficient transport and exchange of gases in the body, particularly between the lungs and tissues.
Here’s how the Haldane Effect works in the context of human physiology:
In Tissues: As cells metabolize nutrients, they produce CO2 as a waste product. This CO2 diffuses into the blood, where it is carried in several forms: dissolved directly in plasma, chemically bound to hemoglobin, and converted into bicarbonate. When hemoglobin releases oxygen to the tissues, its affinity for CO2 increases, allowing the blood to transport more CO2 away from the tissues and toward the lungs for exhalation. This is partly due to the structural changes in hemoglobin when it releases oxygen, which makes it more effective at picking up CO2 and hydrogen ions (H+).
In the Lungs: In the lungs, the process reverses. Oxygen entering the blood from the alveoli binds to hemoglobin, causing hemoglobin to release CO2 and H+. This decrease in CO2 affinity due to the binding of oxygen (oxygenation of hemoglobin) facilitates the release of CO2 into the lungs, from where it is exhaled. The conversion of bicarbonate back into CO2, which then diffuses into the alveoli, is enhanced.
The Haldane Effect emphasizes the reciprocal relationship between oxygen and CO2 transport. It is complementary to the Bohr Effect, which describes how CO2 and H+ affect the oxygen-carrying capacity of hemoglobin (i.e., oxygen affinity decreases in the presence of increased CO2 and H+, facilitating oxygen release in tissues).
Together, the Haldane and Bohr effects are vital for the efficient transport and exchange of oxygen and CO2 between the lungs and tissues, maintaining the balance of gas concentrations necessary for cellular metabolism and function. These mechanisms highlight the intricate regulatory processes underpinning respiratory physiology, ensuring that oxygen delivery and CO2 removal are matched to the body’s metabolic demands.
Haldane Effect Explanation
Let’s begin by taking a look at the following Haldane effect diagram. Let’s suppose I’m moving my arm back and forth. As I move my arm back and forth, the muscle tissue begins to contract, and ATP molecules aren’t produced by those muscle cells.
As cells exercise, they require more Oxygen molecules to produce ATP. So, inside the red blood cells, the Bohr effect causes hemoglobin to unload and release oxygen, which travels into those exercising cells. Inside the cells is a process called aerobic cellular respiration.
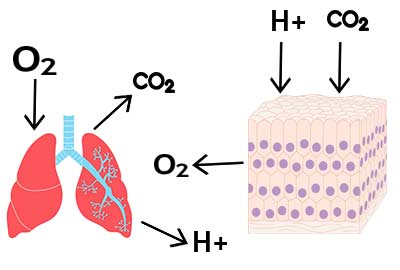
We produce the energy ATP molecules. These ATP molecules are used for muscle contraction and actin contraction. But, Carbon dioxide molecules, a byproduct, are produced. These cannot be used in any helpful way. So, the Carbon dioxide molecules are expelled from the cell and enter the capillary blood plasma. Because Carbon dioxide is nonpolar, only a tiny portion, about 5%, will remain dissolved in the blood plasma.
The majority of it will enter the cytoplasm of the red blood cell. Now, once inside the red blood cell, we have to do it. We must convert the carbon dioxide, a non-polar molecule, into a polar form, bicarbonate. That’s because we want to dissolve the carbon dioxide in the blood. So, most of the carbon dioxide in the red blood cell will follow this reaction pathway.
A special enzyme we call carbonic anhydrase will combine Carbon dioxide and water to form the carbonic acid molecule. Then the Carbonic acid is good. It will dissociate into H+ ions and bicarbonate ions. It is where the Haldane effect takes place.
According to the Haldane effect, Oxygen’s mouth decreases if we decrease oxygen concentration in the red blood cells. Because it travels into the tissue that is exercising tissue, this reduces and increases the affinity of hemoglobin for the H+ ions. If we increase hemoglobin affinity for Hydrogen ions, the hemoglobin will bind these ions. So, the concentration of these free Hydrogen ions will decrease because they will bind to the hemoglobin.
What will happen as we decrease this H+ concentration? Reducing the product concentration will shift the equilibrium toward the right side toward the product side. That means we will produce even more bicarbonate ions as hemoglobin binds hydrogen ions. These bicarbonate ions are the polar form of these carbon dioxide molecules. Ultimately, the Haldane effect increases the bicarbonate ions we can store and dissolve in the blood plasma next to the exercising tissue.
Once again, the Haldane effect is that as we decrease oxygen’s concentration inside the red blood cell as it leaves, it enters our tissue cells. It increases hemoglobin’s affinity to bind hydrogen ions. It shifts the equilibrium to the right side towards the bicarbonate side. That increases the number of CO2 molecules in this form to store and dissolve in the nearby capillary blood plasma.
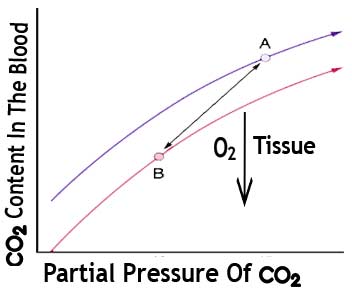
We can also represent this effect graphically. Let’s look at the following graph. The Y-Axis is the carbon dioxide content in the blood dissolved in the blood. The X-axis is the partial pressure of the blood’s carbon dioxide in that region. The curve describes how much carbon dioxide we can fit. How much can we dissolve inside the blood at some given partial pressure of Carbon Dioxide?
As we decrease Oxygen concentration inside the red blood cell, the Haldane effect causes a leftward shift in this curve. But if we consider the Haldane effect, we decrease oxygen in our blood next to the exercising tissue. It shifts the curve to the left to create the new Y coordinate. Notice what that means: we can store even more carbon dioxide inside our blood as oxygen decreases.
We can also use the Haldane effect to describe how the lungs’ alveoli can absorb more Carbon dioxide from the blood plasma. So, the Haldane effect can also explain how oxygen concentration affects carbon dioxide unloading into the alveoli.
Haldane Effect Mechanism
What’s happening inside our lungs? The oxygen moves down its concentration gradient from the alveolus and into the red blood cells inside our lungs. This Haldane effect increases the concentration of oxygen inside our red blood cells. We decrease hemoglobin’s ability to bind Carbon dioxide and H+ ions.
So, as OH binds to hemoglobin, it decreases hemoglobin affinity for h+ in CO2. These two molecules are now released. The carbon dioxide will dissolve and leave the red blood cells in the alveoli. The Hemoglobin also releases Hydrogen ions, which bind to the hemoglobin in this area.
What happens to the hydrogen ions? They essentially recombine with the bicarbonate ions that came from the blood plasma. Remember, these bicarbonate ions dissolve into the blood plasma while the Chloride ions go into the red blood cell, known as the chloride shift.
These move into the cell, and chloride ions leave the cell. This recombines with the H+ to reform the carbonic acid. Then, that breaks down into carbon dioxide. The carbon dioxide then leaves the cell and enters the alveolus. So, the Haldane effect promotes the amount of carbon dioxide the alveolus can absorb.
Significance of Haldane Effect
The Haldane effect is a significant physiological phenomenon that describes the influence of oxygen on carbon dioxide transport in the blood. It has several important implications for gas exchange in the body. Here is the key significance of the Haldane effect:
Oxygen Delivery and Carbon Dioxide Removal: The Haldane effect helps ensure efficient gas exchange in the lungs and tissues. When oxygen binds to hemoglobin in the lungs, it promotes the release of carbon dioxide from the blood, allowing it to be exhaled. In the tissues where oxygen concentration is lower, hemoglobin has a higher affinity for carbon dioxide, facilitating its uptake for transport back to the lungs.
Enhanced Oxygen Unloading: The Haldane effect enhances oxygen unloading from hemoglobin in tissues with a higher carbon dioxide concentration. As carbon dioxide binds to hemoglobin, it causes a conformational change that weakens the binding affinity for oxygen. This facilitates the release of oxygen to the surrounding tissues, allowing for efficient oxygen delivery.
Regulation of pH: Carbon dioxide plays a crucial role in regulating the pH balance in the body. The Haldane effect helps maintain acid-base balance by facilitating carbon dioxide removal from the tissues. As carbon dioxide is released in the lungs, it combines with water to form carbonic acid, which is then converted to bicarbonate ions, helping to regulate blood pH.
Buffering Effect: The Haldane effect also contributes to the blood’s buffering capacity. The formation of bicarbonate ions from carbon dioxide in the blood helps buffer the acidity caused by metabolic processes, maintaining a stable pH within a narrow range.
Understanding the Haldane effect is crucial for comprehending the intricate mechanisms involved in gas exchange and maintaining physiological homeostasis. It illustrates the interdependence of oxygen and carbon dioxide transport and the dynamic nature of gas exchange in the body.
We’ve journeyed through the intricate pathways of our blood, discovering how this vital process ensures that carbon dioxide is efficiently removed, allowing us to breathe freely and keep our bodies functioning optimally. It’s truly remarkable how such complex systems work seamlessly within us every moment. We hope this deep dive has not only enriched your understanding but also sparked a deeper appreciation for the incredible biological symphony happening inside you.
Thank you for joining us on this enlightening adventure. Until next time, keep nurturing your curiosity and marvel at the extraordinary science that makes us who we are. Here’s to breathing easy and exploring more wonders of the human body together!
More Article:
How Do Enzymes Lower Activation Energy & Enzyme Activity
How Do Enzymes Work & Mechanism Of Enzyme Action
How Do Blood Transfusions Work?
References:
Lumb, AB. Nunn’s Applied Respiratory Physiology (5th ed.). Butterworth Heinemann.
Teboul, Jean-Louis; Scheeren, Thomas. “Understanding the Haldane effect.” Intensive Care Medicine.
Siggaard, O; Garby. “The Bohr Effect and the Haldane Effect.” Scandinavian Journal of Clinical and Laboratory Investigation.
Hanson, CW; Marshall BE; Frasch HF. “Causes of hypercarbia with oxygen therapy in patients with chronic obstructive pulmonary disease.” Critical Care Medicine.
Table of Contents
