
A common question in biology is – How do enzymes lower activation energy? If enzymes did not exist, our body would have to rely on them spontaneously during reactions. It would not handle all of the waste products without being broken down. For example, these reactions do not occur as fast enough for our bodies to cope with them alone. Waste products would build up in our bodies, and our digestive system would not be as efficient without enzymes.
Activation energy is needed to break down chemical bonds, allowing the reaction to occur. When an enzyme binds to a substrate, it lowers the substrate molecules’ energy to react to form products. It increases the chance of the reaction occurring and increases the reaction rate.
Not only do they lower the activation energy, but they also increase the possibility of the reaction. If the reaction happens spontaneously, an enzyme’s role is to lower the activation energy needed to start a reaction to proceed quickly without a temperature change.
Make sure that our body does not change in temperature. It is extremely important because, in cells, heat damage can cause a lot of damage to our living tissues. For a chemical reaction to begin an activation, energy is necessary. So, the enzyme does not provide activation energy. It lowers the activation energy needed by bringing the specific molecules together rather than relying on them.
So, enzymes are nature’s exquisite catalysts, integral to the tapestry of life, enabling reactions to occur with remarkable speed and specificity. They ingeniously lower activation energy, the barrier to chemical reactions, allowing life’s processes to flourish even at the mild temperatures within our bodies. This exploration delves into the fascinating mechanisms by which enzymes achieve this feat, illuminating their crucial role in sustaining the biochemical ballet that underpins existence.
What is activation energy?
The amount of energy we have the input for the reaction to convert the reactants to the products. Activation energy differs between the high molecule states and the reactant’s energy. This is called the Gibbs free energy, which Delta G gives.
The activation energy describes how quickly a reaction takes place to be spontaneous. It can have a negative Delta G value but take place very slowly. If a reaction takes place very slowly, what does that mean? It means that it has very high activation energy.
So activation energy is not the same thing as Gibbs free energy. Gibbs free energy describes the difference between the energy of the reactants and the products. But activation energy explains how quickly a reaction takes place. So Gibbs free energy talks about where that equilibrium will be achieved, while activation energy talks about how quickly the equilibrium will be achieved.
How do enzymes lower activation energy?
Enzymes lower the activation energy of a chemical reaction, which is the energy required to initiate the reaction. They facilitate this reduction in activation energy through several mechanisms:
Active Site: Enzymes have a specific active site region where the substrate(s) binds and undergoes the catalytic reaction. The active site provides a precise and complementary environment for the substrate to bind, allowing for optimal positioning of the reactive groups.
Substrate Binding: Enzymes bind to their substrate(s) in a specific and selective manner. The substrate binding to the enzyme’s active site promotes the formation of enzyme-substrate complexes. This binding process brings the reactant molecules into close proximity and proper orientation, enabling more effective collisions and promoting the formation of the transition state.
Transition State Stabilization: Enzymes stabilize the transition state of the reaction. The transition state is an intermediate state with higher energy than the reactants and the products. By binding to the transition state more tightly than the substrates, enzymes lower the energy barrier required to reach the transition state, making it easier for the reaction to proceed.
Active Site Chemistry: The active site of an enzyme often contains specific amino acid residues that participate in the chemical reaction. These residues can act as acids, bases, or catalysts, facilitating the transfer of protons or electrons between the substrate molecules, stabilizing reaction intermediates, or promoting specific chemical transformations.
Microenvironment: Enzymes create a microenvironment within the active site that may differ from the surrounding cellular environment. This microenvironment can have specific properties, such as different pH or metal ion concentrations, which optimize the reaction conditions for the enzyme-catalyzed reaction.
Induced Fit: Enzymes undergo conformational changes upon substrate binding, leading to an induced fit between the enzyme and the substrate. This induced fit can further stabilize the transition state, enhance substrate binding, and facilitate the catalytic reaction.
The enzyme reacts with the substrate and makes the products, and the substrate complements the enzyme’s active site.
Now, we will look at what exactly happens while enzymes and substrates interact. We will look at this interaction’s kinetic data, thermodynamics, and structural aspects.
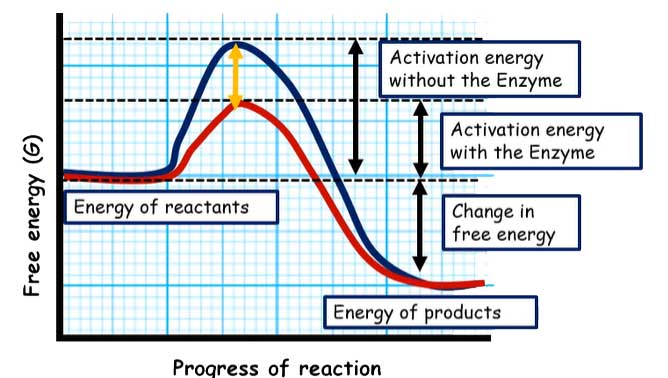
Here is a graph to help us understand how enzymes and substrates work. In the X-axis of the graph, you have the reaction progression. It’s like a time when you have free energy change in the Y-axis.
Without any enzyme, adding some substrate would eventually become a product, but it would take a lot of time. The graph looks like this: you have the reactants’ energy as the initial part, and then eventually, it would get converted into the product.
The activation energy is the difference between the reactant and the transition state. So, activation energy without the enzyme looks like the black curve. The graph looks like the red curve if you have an enzyme that can catalyze this reaction.
As you can notice, the activation energy has been reduced to start the reaction with the enzyme. This reduction in the activation energy is the key aspect of the enzyme-substrate reaction because it reduces the active activation energy. So, what is activation energy?
- The difference between the ground state’s energy levels and the transition state can be called activation energy.
The enzyme and enzyme-substrate interaction decreases the activation energy and reaction rate.
- The enzyme does not change the equilibria. It is one of the most critical aspects of how enzymatic reactions work.
Now let’s try to understand how enzyme specificity is brought about in more detail.
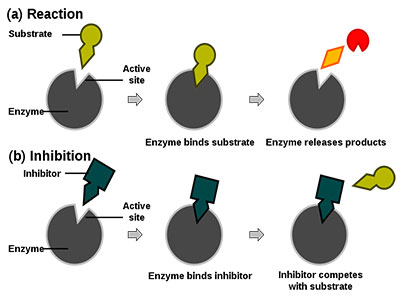
Here is an enzyme binding to this particular substrate, but this specific enzyme will not bind with another substrate. There is a mismatch, or this specific substrate does not fit into the enzyme’s active site. To understand these aspects, we must look at the structural elements of these enzyme-substrate interactions and develop an X-ray crystallography technique.
Structural biology has flourished. It looks at the enzyme substrate-bound or enzyme structure individually in an unbound situation, crystallizes both confirmations, and follows a different crystallographic technique. Scientists have found out how exactly enzyme-substrate works. They discovered several principles associated with the enzyme’s catalytic power and specificity. So we are going to look at that in a moment.
There are two key principles:
- First, there is something called binding energy, which augments the enzyme-substrate interaction.
- The stereospecificity of an enzyme occurs due to the 3d conformation of the enzyme’s active site.
Let’s talk about binding energy. So, the energy derived from the enzyme-substrate interaction may be a weak interaction known as the binding energy.
Binding energy is the primary energy source used by the enzyme to lower the activation energy. So, the key aspect of the enzyme-substrate reaction is that the enzyme reduces the activation energy for this reaction, thereby augmenting the rate. The binding energy provides this. The substrate is bound with an enzyme. In the active site, there are several interactions. Non-covalent or equivalent interactions are forming in the active area.
For example, a hydrogen bond is formed between two particular residues between these two substrates and enzymes. That bond formation energy contributes to the binding energy. There is not only one interaction. There could be multiple different small interactions. Some are hydrogen bonds, and some may be hydrophobic or Vander Waals interactions.
All summation of these bond formation energies would contribute to the binding energy, with excellent important implications. Now, that gives the enzyme the catalytic power. These weak interactions are optimized in the reaction’s transition state. It must be remembered that enzymes conformation the active site conformation to complement the substrate, not in the ground state. But in the transition state, let us try to visualize this process.
It is the enzyme. Its active site is not complementary to the substrate’s stereospecificity. So, the substrate first binds a site away from the active site, and multiple small interactions occur between the enzyme and substrate. But while the enzyme-substrate reaction is transitioning, the active site’s confirmation also changes. The initial weak interactions between the enzyme and substrate induce it. It changes the active site’s conformation such that the substrate fits nicely in the enzyme’s active site.
It is the importance of binding energy. We learned from this that there is a loose binding of enzyme and substrate in the ground state, but the form binding only occurs in the transition state, which augments this process and lowers the activation energy. The binding energy provides the energy to lower the activation energy. Thereby, the reaction can go on, and products are formed.
How do enzymes lower activation energy A Level Biology?
Understanding how enzymes lower activation energy to catalyze reactions is fundamental in A-level biology. Enzymes are biological catalysts that speed up biochemical reactions by providing an alternative reaction pathway with a lower activation energy than the reaction proceeding without an enzyme. Here’s how they achieve this:
- Formation of Enzyme-Substrate Complex
Enzymes have a specific region called the active site, which is complementary in shape to the substrate (the molecule upon which an enzyme acts). An enzyme-substrate complex is formed when the substrate binds to the active site. This specificity is due to the precise arrangement of atoms in the active site, allowing for the efficient binding of the substrate through various interactions such as hydrogen bonds, ionic bonds, and hydrophobic interactions.
- Transition State Stabilization
A reaction’s activation energy is required to reach the transition state, where bonds in the reactants are weakened and ready to form new bonds to become products. Enzymes lower the activation energy by stabilizing this transition state more effectively than the substrate alone. This stabilization involves the enzyme changing shape slightly to better accommodate the substrate(s) in a process known as induced fit, which further reduces the energy required to reach the transition state.
- Providing an Optimal Microenvironment
The enzyme’s active site can also provide an optimal microenvironment for the reaction. For example, suppose the reaction requires a lower pH than the surrounding environment. In that case, the active site has acidic or basic amino acid residues that provide the necessary conditions without affecting the pH of the cell or bodily fluid in which the enzyme operates.
- Orienting Substrates Correctly
By binding to its substrates, an enzyme orientes them precisely so that reactive groups are brought into alignment, making reactions more likely to occur. This reduces the entropy (disorder) of the system and lowers the energy needed for the reactants to collide in the correct orientation for a reaction to occur.
- Inducing Strain on the Substrates
When substrates bind to the active site of an enzyme, the enzyme may induce strain on the bonds within the substrate, making them easier to break. This strain pushes the substrate closer to the transition state, reducing the energy required for the reaction to proceed.
- Temporary Covalent Bond Formation
Some enzymes work by forming a temporary covalent bond with the substrate. This lowers the activation energy required for the reaction as it may make it easier to break other bonds in the substrate or facilitate the transfer of atoms or groups within the substrate.
By lowering the activation energy, enzymes allow reactions to occur rapidly and at relatively low temperatures, which is crucial for sustaining life’s processes. This capacity of enzymes to catalyze reactions efficiently and specifically under mild conditions compatible with life distinguishes them as vital biological catalysts in a wide array of physiological processes, from digestion and metabolism to DNA replication and repair.
How do enzymes speed up reactions?
Enzymes speed up chemical reactions by lowering the activation energy required for those reactions to proceed, thereby increasing the rate at which they occur. This fundamental property allows biological processes to happen quickly enough to sustain life. Here’s a breakdown of how enzymes achieve this acceleration:
- Lowering Activation Energy
Activation Energy: This is the energy barrier that must be overcome for reactants to be transformed into products. Enzymes lower this barrier, making it easier for the reaction to occur.
Transition State Stabilization: Enzymes stabilize the transition state of a reaction more effectively than the reactants can on their own. This stabilization requires less energy to reach the transition state, effectively lowering the activation energy.
- Forming Enzyme-Substrate Complexes
Specific Binding: Enzymes have a unique active site that specifically binds to its substrate(s), forming an enzyme-substrate complex. This specificity ensures that enzymes catalyze only specific reactions.
Induced Fit: The induced fit model suggests that the binding of the substrate induces a conformational change in the enzyme, optimizing the interaction between the enzyme and substrate and further lowering the activation energy.
- Providing an Optimal Reaction Environment
Microenvironment: The active site of the enzyme can provide a microenvironment more conducive to the reaction, such as a specific pH or ionic strength, different from the broader cellular environment.
Proximity and Orientation: Enzymes bring substrates together in the optimal orientation for the reaction to occur, which increases the rate at which the reactant molecules collide with the correct orientation.
- Inducing Strain on Substrates
Enzymes can induce strain on the bonds within the substrate(s), making them more reactive and closer to the transition state. This strain helps decrease the activation energy needed for the reaction.
- Catalytic Strategies
Acid-Base Catalysis: Enzymes can donate or accept protons (H+) to/from the substrate to facilitate the breaking and forming of bonds.
Covalent Catalysis: Temporary covalent bonds form between the enzyme and substrate, lowering the activation energy required for bond-breaking in the reaction.
Metal Ion Catalysis: Enzymes use metal ions to help stabilize negative charges on the reaction’s transition state or to act as an electrophile to attract electrons from the substrate.
- Reducing the Reaction Pathway
Enzymes provide an alternative reaction pathway with a lower activation energy, allowing the reaction to proceed more rapidly without the enzyme.
Application and Importance
Metabolism: Enzymes catalyze virtually all types of biochemical reactions involved in metabolism, allowing organisms to build up and break down molecules efficiently.
Regulation: The activity of enzymes is finely regulated (inhibited or activated), allowing cells to control biochemical pathways in response to changes in the cell’s environment or signals from other cells.
By efficiently catalyzing chemical reactions at relatively low temperatures and within narrow pH ranges, enzymes allow complex biological processes to occur with remarkable specificity and speed, underscoring their essential role in sustaining life.
How do enzymes lower the activation energy of chemical reactions?
Enzymes lower the activation energy of chemical reactions through several sophisticated mechanisms, enabling these reactions to occur more quickly and efficiently than without enzymes. This capacity is crucial for sustaining the myriad of life’s biochemical processes under the mild conditions characteristic of living organisms. Here’s how enzymes accomplish this remarkable feat:
- Formation of Enzyme-Substrate Complex
Specific Interaction: Enzymes have a unique active site that is complementary in shape to their specific substrate(s). The binding of the substrate to the active site forms an enzyme-substrate complex, which is the first step in the catalytic process.
Induced Fit: Upon substrate binding, the enzyme undergoes a conformational change that more snugly fits the substrate (induced fit), which helps to position the substrate optimally for the reaction.
- Transition State Stabilization
Lower Activation Energy: The activation energy is the energy required to reach the transition state, a high-energy, unstable state necessary for the reactants to convert into products. Enzymes lower this energy barrier primarily by stabilizing the transition state, making it easier and thus faster for the substrate(s) to reach this state.
Stabilization Mechanisms: This stabilization can involve non-covalent interactions such as hydrogen bonds, ionic bonds, and van der Waals forces between the enzyme and the substrate. By stabilizing the transition state more effectively than the substrate alone, the enzyme reduces the amount of energy needed to transform the substrate into the product.
- Providing an Optimal Reaction Environment
Microenvironment Modification: An enzyme’s active site can create a more favorable microenvironment for the reaction than the general cellular environment. For example, if the reaction requires a lower pH, the active site might provide this acidic condition locally without affecting the cell’s overall pH.
- Orienting Substrates Correctly
Proximity and Orientation Effects: By binding substrates, enzymes bring them into close proximity and in the correct orientation to react. This reduces the entropy (disorder) of the system and decreases the energy required for the molecules to collide in the correct orientation for a reaction to occur.
- Inducing Strain on Substrates
Strain Induction: Enzymes can induce strain on the bonds within the substrate, making them more susceptible to breaking. This bending or stretching of bonds can push the substrate closer to the transition state.
- Temporary Covalent Bond Formation
Covalent Catalysis: Some enzymes catalyze reactions by forming temporary covalent bonds with their substrates. This lowers the activation energy by providing a more direct pathway for transferring electrons or groups within the substrate molecules.
- Catalytic Strategies
Acid-Base Catalysis: Enzymes can donate or accept protons to or from the substrate, facilitating the breakage and formation of bonds.
Metal Ion Catalysis: Metal ions in the active site can help stabilize negative charges on the transition state or participate directly in the catalysis by acting as electrophilic catalysts or facilitating nucleophile formation.
By employing these mechanisms, enzymes significantly increase the rate of biochemical reactions, ensuring that vital physiological processes occur rapidly enough to meet the demands of living organisms. This efficiency is a testament to the evolutionary refinement of enzymes as catalysts tailored to life’s complexity.
Frequently asked questions
How does the enzyme affect the activation energy?
The enzyme does not change the Gibbs free energy of the reaction. It does not affect the energy of the reactants and the products. So their difference in Delta G is the same.
It remains unchanged when the enzyme acts on that chemical reaction but affects activation energy. The enzyme typically does that. It lowers that transition state’s energy by lowering its energy.
– Enzymes do not affect equilibrium. They do not affect the Gibbs free energy of that reaction. The reactants’ thermal free energy and the free energy of products remain unchanged for any catalyzed reaction.
However, the enzymes stabilize the transition state and lower its energy. They lower the energy of that transition state, decreasing the activation energy. It speeds up that chemical reaction.
When they act on chemical reactions, enzymes do not change the Gibbs free energy, which means they do not increase or decrease how many products are formed at the end of that reaction. But they allow equilibrium to be achieved quicker by increasing the rate by reducing that activation energy.
In unraveling the mystery of how enzymes lower activation energy, we’ve journeyed through the cellular microcosm, revealing the elegance and efficiency of these biological catalysts. Their ability to facilitate vital reactions swiftly and precisely underscores the harmony of biological systems, ensuring the continuity of life’s myriad functions. Armed with this knowledge, we gain a deeper appreciation for the biochemical underpinnings of life and a glimpse into the potential for harnessing these natural wonders in medicine, industry, and beyond.
Read More:
How Do The Neurotransmitters Work?
How Does pH Affect Enzyme Activity?
References:
Stryer L, Berg JM, Tymoczko JL. Biochemistry (5th ed.). San Francisco: W.H. Freeman.
Murphy JM, Farhan H, Eyers. “Bio-Zombie: the rise of pseudoenzymes in biology.” Biochem Soc Trans.
Radzicka A, Wolfenden. “A proficient enzyme.” Science.
Callahan BP, Miller BG. “OMP decarboxylase—An enigma persists.” Bioorganic Chemistry.
Williams HS. A History of Science: in Five Volumes. Volume IV: Modern Development of the Chemical and Biological Sciences.
Table of Contents
