In chemistry, pH calculates the relative proportions of hydrogen plus (H+) and hydroxide minus ions (OH−). Simply put, pH is a substance’s acidity or acidicity when a solution is acidic, such as your stomach’s contents. It means many hydrogen ions and atoms floating around your stomach have lost their electrons and become positive. If you compare that solution to a glass of water, the water contains far fewer hydrogen protons, mostly water molecules.
Enzymes speed up chemical reactions. They have a groove on their surface called the active site to do this. The enzyme breaks down the substrate into products. The substrate must fit perfectly into the lock and key theory active site. Enzymes are very fussy molecules with the right pH to work in a good environment. We will describe the effect of pH and enzyme activity by looking at the graph.
Dive into the molecular world where the subtle shifts in pH can dramatically alter the course of enzymatic actions, steering the biological processes that sustain life. This exploration deciphers how pH levels influence enzyme activity, impacting their structure, function, and the intricate dance of biochemical reactions within organisms.
What is pH?
The pH of a solution is the measure of hydrogen ion concentration. Each enzyme has an optimum pH. Many ionic bonds hold the tertiary structure of an enzyme and, therefore, the active site’s shape.
Each enzyme has a range of pH values called its optimum pH, at which the reaction rate is catalyzed fastest. The reason pH has such an important effect on enzymes is their proteins. The change in pH will change the bonding patterns; as a result, increased changes from the optimum pH will change the shape of the active site.
When the temperature rises too high in enzyme-controlled reactions, the change in the active site is irreversible. It is permanent. So, it causes a far more drastic change in the reaction rate.
How Does pH Affect Enzyme Activity?
pH can significantly affect enzyme activity. Enzymes are biological catalysts that facilitate chemical reactions in living organisms. They have an optimum pH range, where their activity is maximized. Deviations from this optimal pH can impact enzyme activity in the following ways:
Effect on Enzyme Structure: Enzymes have a specific three-dimensional structure crucial for their activity. pH can alter an enzyme’s ionic and hydrogen bonding interactions, causing changes in its overall structure. If the pH deviates significantly from the optimum range, the enzyme’s structure can be disrupted, leading to denaturation or loss of its catalytic activity.
Ionization of Substrate and Active Site: The pH affects the ionization states of both the enzyme and its substrate. The active site of an enzyme often contains amino acid residues with ionizable groups that participate in catalysis. When the pH is not optimal, the ionization of these groups can be disrupted, affecting the binding of the substrate to the active site and the subsequent catalytic reactions.
Optimal pH Range: Each enzyme has an optimal pH range exhibiting maximum activity. This pH range depends on the specific enzyme and the environment in which it functions. Deviating from this optimal pH can decrease enzyme activity. For example, an enzyme with an optimal pH of 7 (neutral pH) may have reduced activity in a more acidic (lower pH) or alkaline (higher pH) environment.
Influence on Charge Distribution: pH affects the distribution of charges on the enzyme’s active site and the substrate. The active site’s charge distribution is crucial for substrate binding and catalysis. Deviations from the optimal pH can disrupt the charge interactions between the enzyme and substrate, impacting the enzyme’s ability to bind the substrate and perform catalytic reactions.
The pH measures the acidity or basicity of a substance or a solution. Our stomach produces powerful acids that help us digest our foods. This acid helps us digest but also kills harmful microorganisms we eat from our food.
Our blood has a pH of 7.4, so that’s quite neutral and very similar to water. One of the few basic foods we eat is baked beans; we all know you may produce more gas.
That’s because of the effect of basic food on our digestive system. It doesn’t mean that it’s bad for us. It’s still healthy to eat acids. What are acids? They are substances with hydrogen ions and have a pH lower than 7.
pH Scale:
- pH 7 – Neutral.
- pH 0 to 6 – Acidic
- pH 8 to 14 – Alkaline
If you go above or below the pH optimum for each enzyme, you can see that the reaction rate decreases. This is because the enzyme denatures. It changes shape above or below the optimum pH.
- If you have below 9 pH, the enzyme’s active site denatures. So, above pH 7, the active site is denaturing.
- The active site will denature if you have below pH 7 in more acidic conditions. Therefore, the substrate can no longer bind, the product form decreases, and the reaction rate decreases.
Results: The enzyme’s active site is natural if you increase or decrease the pH above or below the optimum. The active site changes shape, and the substrate no longer binds. Therefore, less product is formed, and the reaction rate will decrease. The rate of reaction will decrease.
Enzymes work best with a narrow pH range. Any variation above or below a specific level reduces their rate of activity.
Examples of enzymes: Pepsin, Trypsin, Amylase, Rennin, etc.
pH for Pepsin: Pepsin is a powerful enzyme that digests proteins in the stomach. It digests meat, eggs, seeds, or dairy products and breaks them into peptides. These peptides are absorbed from the intestine to the bloodstream or broken down further by pancreatic enzymes such as trypsin.
Its optimal pH is 2. Its pH is around 2 because the stomach’s pH is about 1.5 to 3.
pH for Trypsin: Trypsin works at six to seven alkalinity levels or acidity outside the optimum pH—enzymes in the digestive tract function in an acidic or alkaline environment.
pH for Amylase: It breaks down starch and best works in a weak acid condition. So, its pH is around 6.7, but it can also range up to pH 7, which is neutral.
The action of amylase on starch stops when the food passes into the stomach. It is because the low pH of the gastric juice makes it inactive. So, the amylase cannot work or perform its function with a pH of 1.5 to 3.
pH for Rennin: The digestive protein enzyme rennin is found in gastric juice in the stomach function. It’s turning milk, a liquid, into a solid. The purpose of this is so that you’re able to absorb all the nutrients in that solid.
If it were to stain as a liquid, it would pass through the body, and you wouldn’t be able to absorb the proteins and nutrients from the milk. A commercial form of renin is called rennet.
Rennin has an optimum pH of 3.4. At values pH above 7, it loses activity rapidly.
The graph of pH affects enzyme activity.
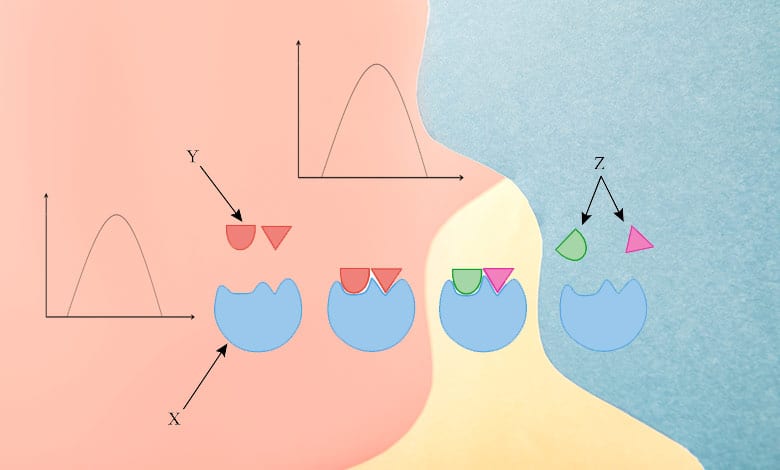
Here is a graph of increasing enzyme activity on the Y-axis against increasing pH on the X-axis. The shape of the curve denotes the increase in enzyme activity. It reaches a maximum and then decreases. What’s happening at points X, Y, and Z?
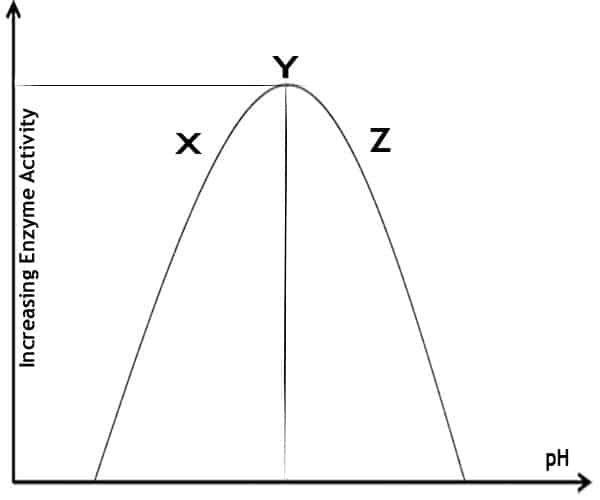
- At point X, which is low pH. The enzyme is protonated, which means it has a positive charge. Therefore, the substrate cannot bind effectively at the active site. So at low pH, the enzyme is protonated.
- At point Y, which is the optimum pH. The substrate can bind effectively at the active site.
- The enzyme is deprotonated at point Z, which is high in pH. It means it has a negative charge. Therefore, this substrate is enabled to bind effectively at the active site.
How does pH affect the protons?
- Increasing the ph level decreases the excess protons in the solution. It means deprotonating the solution.
- When the ph level decrease, it adds excess protons to the solution. It means protonating the molecules that are there in the solution.
Experiments/Practical of the pH affect enzyme activity
Objective: To investigate how different pH levels affect the activity of an enzyme.
Hydrogen peroxide is very toxic to both the body and the environment. It needs catalase to break it down into water and oxygen. Catalase is found in all organisms that breathe oxygen.
This enzyme facilitates the decomposition of hydrogen peroxide into water and oxygen, a toxic byproduct of the body.
The optimum pH for human catalase is approximately seven and has a fairly broad maximum. So the rate of reaction does not change too much at levels beyond like
6.8 and 7.5.
Sources of catalase include:
- Sliced raw potato.
- Ground meat.
- Liver.
Hypothesis: When catalase, an enzyme, is added to a medium higher or lower pH outside its optimum pH range, the enzyme will denature. Therefore, it does not perform its function of converting hydrogen peroxide into water and oxygen.
Materials:
- Hydrogen peroxide (3%).
- Paper immersed with catalase.
- pH 4, 7, and 10 solutions.
- Beakers and a stopwatch.
Method:
- Place the catalase paper at pH 4
- Using a stopwatch to determine how long it takes for the reaction to take place.
- Repeat steps 1-2 with catalase in solutions of pH 7 and pH 10.
- Record results in a table and draw a graph of the results.
Data Table:
| pH | Trial 1 (mm) | Trial 2 (mm) | Trial 3 (mm) | Average Bubble Height (mm) |
|---|---|---|---|---|
| 4 | 5 | 4 | 6 | 5 |
| 6 | 15 | 14 | 16 | 15 |
| 7 | 22 | 23 | 21 | 22 |
| 8 | 18 | 19 | 17 | 18 |
| 10 | 10 | 9 | 11 | 10 |
Results:
- For pH 4, There was no reaction. The enzyme has denatured.
- For pH 7, The Fastest reaction is at 90 seconds to complete.
- For pH 10, the Slowest reaction is at 5 minutes to complete.
The pH 10 still achieved a reaction. The enzyme did perform at this pH level. The enzyme may be able to withstand varying pH levels. However, it performs best at pH 7 but not at pH 4.
Record all results in a table and draw a graph of the results.
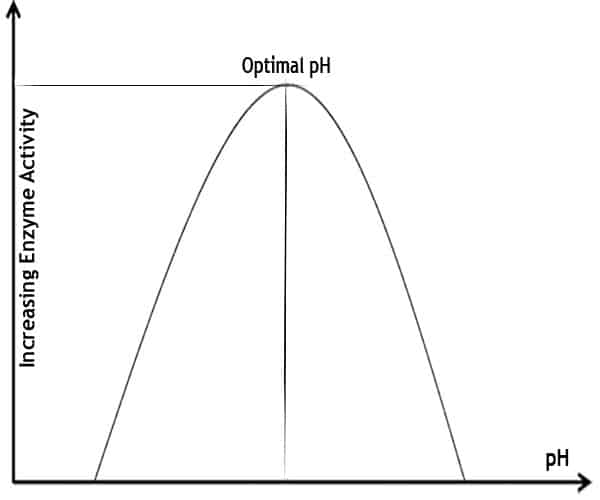
The enzyme may be able to withstand varying pH levels. However, it performs best at pH 7 but not at pH 4. Each enzyme will work at a specific level of pH.
How does pH affect enzyme structure?
The pH level can significantly impact an enzyme’s structure and, as a result, its activity. Enzymes are proteins, and their function depends on their three-dimensional shape, especially the shape of their active site, where the substrate binds. Here’s a breakdown of how pH influences enzyme structure:
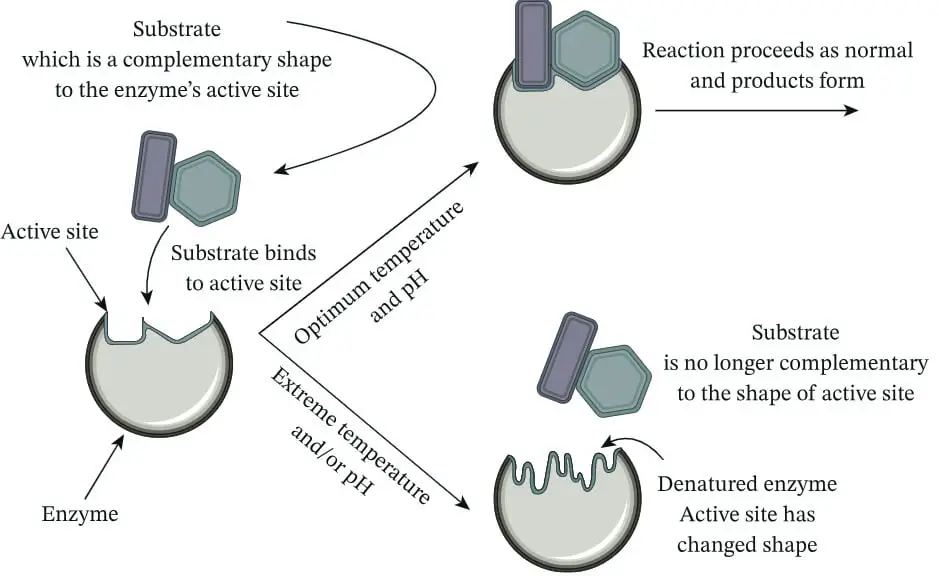
- Protein Structure Basics:
Enzymes are composed of long chains of amino acids that fold into a specific three-dimensional shape.
This shape is held together by various chemical bonds and interactions, such as hydrogen bonds, ionic bonds, and disulfide bridges.
The specific folding pattern of the enzyme determines the shape of the active site, which must match the shape of the substrate for a reaction to occur.
- pH and Ionic Bonds:
The side chains of amino acids in enzymes can be charged (positive or negative) depending on the pH of their environment.
At an enzyme’s optimum pH, the charges of these side chains help maintain the proper structure of the enzyme by forming ionic bonds.
When the pH is too low (acidic) or too high (basic), it can alter the charges of these side chains.
This change in charge can disrupt the ionic bonds, causing the enzyme to unfold or change shape. This is known as denaturation.
- Hydrogen Bonds and pH:
Hydrogen bonds are also crucial for maintaining an enzyme’s structure.
pH changes can add or remove protons (H+ ions) from amino acid side chains, affecting hydrogen bonding.
When these bonds are altered, the precise folding of the enzyme can change, leading to a loss of its active site’s shape and, therefore, its ability to bind to the substrate.
- Active Site Shape and pH:
The active site of an enzyme is where the chemical reaction occurs, and its shape is extremely sensitive to pH changes.
If the pH moves far from the enzyme’s optimal range, the shape of the active site may distort or change, preventing the substrate from binding effectively.
This leads to a decrease in enzyme activity because the enzyme can no longer facilitate the reaction as efficiently or, in extreme cases, not at all.
- Denaturation at Extreme pH Levels:
At extremely high or low pH levels, the enzyme’s structure may become irreversibly altered.
Denaturation is when the enzyme loses its specific three-dimensional structure and unfolds.
A denatured enzyme is usually inactive because the active site is no longer intact and cannot bind the substrate.
Example: Catalase and pH
For example, catalase (an enzyme that breaks down hydrogen peroxide) has an optimum pH around 7.
At this pH, the enzyme’s structure is stable, and the active site is perfectly shaped to interact with hydrogen peroxide.
If the pH drops to 4 (acidic) or rises to 10 (alkaline), the changes in hydrogen ion concentration can disrupt the bonds holding the enzyme’s structure, altering the active site and reducing its ability to bind hydrogen peroxide effectively.
What happens to an enzyme when the pH increases?
The effect of pH on an enzyme’s activity is crucial because enzymes are highly sensitive to the pH of their environment. Each enzyme has an optimal pH range within which it exhibits peak activity. Deviations from this optimal pH can significantly impact the enzyme’s function in several ways:
- Optimal pH Range
Every enzyme works best at a specific pH value or within a narrow pH range. This optimal pH corresponds to the environment in which the enzyme typically operates, such as the stomach’s acidic environment for pepsin or the nearly neutral pH of the cytoplasm for many cellular enzymes.
- Effects of pH Increase
When the pH increases beyond an enzyme’s optimal range (becoming more basic or less acidic), several changes can occur:
Denaturation: An increase in pH can lead to denaturation of the enzyme. Denaturation involves disrupting the enzyme’s secondary and tertiary structure, leading to a loss of its active site’s specific shape and, consequently, its ability to bind to substrates.
Altered Charge Properties: Enzymes comprise amino acids, which can accept or donate protons (H+ ions) depending on the pH. Changes in pH can alter the charge on the enzyme’s active site, affecting its ability to bind to the substrate.
Reduced Activity: Even before denaturation occurs, slight changes in pH can reduce the enzyme’s activity by altering the enzyme and substrate’s binding efficiency or the rate at which the product is formed.
Substrate Affinity Changes: The enzyme’s affinity for its substrate may change with pH, affecting the reaction rate.
- Reversibility
The effects of a pH increase on an enzyme can be reversible or irreversible, depending on the degree of change and the duration. Slight or short-term changes in pH might allow the enzyme to regain its activity once the pH returns to its optimal range. However, significant alterations in pH or prolonged exposure to suboptimal pH conditions can lead to irreversible enzyme denaturation.
- Enzyme Stability
Stability is another aspect affected by pH. Enzymes may become more unstable and prone to degradation when outside their optimal pH range.
- Application in Industry and Research
Understanding the pH sensitivity of enzymes is exploited in various industrial and research applications to regulate enzyme activity. This includes adjusting the pH of the environment to activate, inhibit, or modulate the action of enzymes in processes such as brewing, food manufacturing, and pharmaceuticals.
In summary, increasing pH away from an enzyme’s optimal range can decrease activity, potentially leading to denaturation and a loss of function. The specific effects depend on the enzyme, how far the pH is from the optimum, and the duration of exposure to the altered pH conditions.
How does concentration affect enzyme activity?
The concentration of the enzyme and the substrate can significantly affect enzyme activity, influencing the rate at which a biochemical reaction proceeds. Here’s an overview of how variations in concentration impact enzyme activity:
- Enzyme Concentration
Increasing the enzyme concentration generally increases the reaction rate up to a point. This effect is due to increased active sites to convert substrate molecules into products. However, this relationship holds true only if the substrate concentration is abundant; at some point, the reaction rate plateaus because the substrate becomes the limiting factor—there are more enzymes than the substrate can occupy, leading to some enzymes not being used.
Low to Moderate Increases: With more enzyme molecules available, more substrate molecules can be converted to product per unit time, increasing the reaction rate.
Plateau: Once all substrate molecules are bound and converted to a product, adding more enzymes no longer increases the reaction rate because the substrate concentration becomes the limiting factor.
- Substrate Concentration
The effect of substrate concentration on enzyme activity follows a characteristic pattern, often described by the Michaelis-Menten kinetics:
Low Substrate Concentration: An increase in substrate concentration at low substrate concentrations leads to a proportional increase in the reaction rate. This is because many active sites are available, and the likelihood of substrate molecules colliding with and binding to active sites is low.
Moderate Increases in Substrate: As the substrate concentration increases, the reaction rate continues to rise but at a slower pace. More active sites are occupied, and the enzyme works closer to its maximum capacity.
Saturation Point: Eventually, a point is reached where the substrate occupies all active sites of the enzyme molecules. This is known as enzyme saturation. Beyond this point, increases in substrate concentration do not increase the reaction rate because the enzyme is working at its maximum capacity (Vmax).
- Effects of Exceeding Saturation
Once the enzyme is saturated with the substrate, adding more enzyme molecules is the only way to increase the reaction rate further.
- Enzyme Inhibition
Both enzyme and substrate concentrations can also influence the effectiveness of enzyme inhibitors. Competitive inhibitors, which compete with the substrate for active sites, can be overcome by increasing the substrate concentration. However, non-competitive inhibitors affect the enzyme activity regardless of substrate concentration because they bind to other parts of the enzyme, altering its activity.
- Practical Implications
Understanding how concentration affects enzyme activity is crucial in various fields, such as biochemistry, medicine, and industrial processes. It helps design drugs that target specific enzymes, optimise industrial biocatalysts, and understand metabolic pathways and their regulation within cells.
In summary, the concentration of enzymes and substrates is critical in determining the rate of enzyme-catalyzed reactions. The relationship between these concentrations and reaction rates is vital for understanding biological systems and applying enzymes in industrial and medical settings.
Last Words
We gain not only a deeper understanding of biochemical principles but also an appreciation for the delicate balance that enzymes navigate. This knowledge opens doors to innovations in medicine, agriculture, and biotechnology, where controlling pH can optimize enzyme efficiency, heralding new solutions to age-old challenges.
Learn More:
How Do Enzymes Lower Activation Energy – Enzyme Activity
References:
Stryer L, Berg JM, Tymoczko JL. Biochemistry (5th ed.). San Francisco: W.H. Freeman.
Murphy JM, Farhan H, Eyers PA. “Bio-Zombie: the rise of pseudoenzymes in biology.” Biochem Soc Trans.
Radzicka A, Wolfenden R. “A proficient enzyme”. Science.
Table of Contents