
From a young age, everyone is taught that water is the enemy of fire. Everyone’s taught that water kills fire, but no one goes on to explain it further. For various reasons, water is unsuitable for all fires, like oil or chemical fires. First, we must discuss the fire triangle to answer why water kills fire.
There are three major requirements that fire has. We need oxygen, fuel, and heat for any fire to occur. In every case, a fire is extinguished, and at least one of these elements is removed.
So, if we use water to extinguish a fire, which one of these requirements are we taking away? When asked this question, most people say it blocks or takes away oxygen. But what it’s doing is it’s taking away the heat.
So if you’re only interested in the fast and quick answer, you have it. It removes the heat. Though that’s a superficial answer, I would like to get into more detail and explain why blocking oxygen is a good answer.
Why does water kill fire? (The reasons for fire extinguishing by water)
Water is commonly used to extinguish fires because it has several properties that make it effective in firefighting:
Heat Absorption: Water has a high specific heat capacity, meaning it can absorb significant heat energy. When water is applied to a fire, it absorbs heat from the fire, reducing the temperature of the burning material. This heat absorption helps to cool down the fuel and the surrounding area, preventing the fire from spreading further.
Evaporation: Water absorbs heat and undergoes a phase change from liquid to vapor when it comes into contact with fire. This phase change requires a large amount of energy drawn from the fire and further cools the burning material. The vaporization of water also displaces oxygen, reducing the availability of oxygen necessary for the fire to sustain itself.
Dilution: Water can dilute flammable substances and reduce their concentration, making it more difficult for the fire to sustain itself. By dispersing water over the burning material, it can decrease the fuel concentration, reducing the likelihood of ignition or slowing down the burning process.
Heat Barrier: Water can create a physical barrier between the fire and the surrounding combustible materials. Applying a layer of water can prevent flames and heat from reaching nearby objects, helping to contain the fire and prevent it from spreading.
Activation energy: A lot of reactions generally require something called activation energy. It means that a certain amount of energy is required for the reaction to start to get a lot of reactions going. We often need to put in some initial energy to break molecular bonds.
Once these bonds are broken, the atoms of the reactants are free to rearrange. In the case of fire, the atoms rearrange and form more stable products than the reactants. More stable is a different way of saying that there’s less energy associated with the bonds between the products than the reactants.
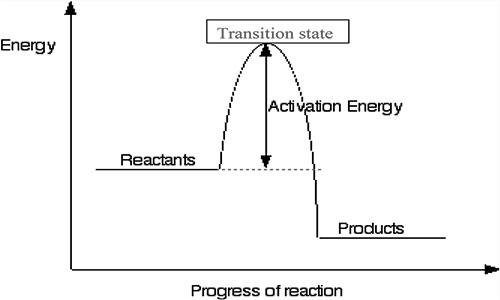
This difference in energy between the products and the reactants is released into the environment, usually in the form of heat. From this diagram, it’s essential to notice that the released energy differs entirely from the activation energy. Even if the reaction releases a vast amount of heat and energy, we still need to start by giving it a little kick-start of activation energy.
In the case of fire, we usually react with carbon-based fuel with oxygen to form very stable carbon dioxide and water. This conversion results in the less stable fuel to the very stable carbon dioxide, and water results in the release of energy. To get any fire going is to overcome the activation energy. This activation energy barrier is why most things don’t burst into flames when they touch air. Once the fire gets started, it becomes self-sustaining.
One fuel molecule reacting with oxygen releases enough energy to cover the activation energy of the molecules around it. So only one molecule needs to react to get multiple molecules around it. It is one reason why fire can easily get out of control.
But what does that have to do with extinguishing a fire? If we could absorb enough energy that the fire could no longer provide its activation energy, it would no longer be self-sustaining and die out. It might not be immediately obvious, but we’re doing this with water.
How does water extinguish fire?
Water has two major things going for it. It’s completely non-flammable and takes a huge amount of energy to vaporize. Water is flammable because it’s one of the final combustion products and can’t be oxidized further by oxygen. Oxygen and water would only reform oxygen and water. This reaction is nonproductive and doesn’t happen. The boiling point of water is much lower than the temperature of a fire.
So when put on one, it immediately starts to boil off. The energy required to convert water from a liquid to a vapor is surprisingly tremendous. The temperature of the average wood fire is between 400-600°C. I was surprised that it could ignite at temperatures as low as 150°C.
Even though this is surprisingly low, the fire has to maintain at least a temperature of about 150°C to maintain ignition. In other words, it must reach a temperature of at least 150°C to overcome its activation energy.
If water is added to this wood fire, it would quickly absorb all the energy and heat around it and convert to steam. The conversion of liquid water to steam takes place at around 100°C and takes a lot of energy. So, the fire’s heat is diverted into converting the water to steam. Eventually, we’ll add enough water that it’s too much for the fire to boil off.
Once we do this, the wood’s or the fuel’s temperature will be 100°C or less. It is at least 50°C less than the activation temperature. Because the fire is no longer contributing the heat necessary to reach its activation temperature. It pretty much dies out. The water brings the temperature of everything. It touches down to about 100°C and absorbs huge amounts of energy to be converted to steam.
If a fire can’t provide enough energy to boil off the water and bring the fuel back up to its activation energy, the fire will be extinguished.
Read more similar topics:
Why Can Not We Use Sea Water To Put Out Fires?
Why Does Water Taste Better At Night?
Why Does Bottled Water Taste Bad?
Why Does A Object Look Bent In Water?
Can Water Boil And Freeze At The Same Time?
How Does Water Get Into Coconut?
References:
Haung, Kai. Population and Building Factors That Impact Residential Fire Rates in Large U.S. Cities. Applied Research Project. Texas State University.
Karki, Sameer. “Community Involvement in and Management of Forest Fires in South East Asia”. Project FireFight South East Asia.
Espenson, James. Chemical Kinetics and Reaction Mechanisms. McGraw-Hill. ISBN 0070202605.
“Activation Energy and the Arrhenius Equation – Introductory Chemistry- 1st Canadian Edition”.
Kagan, Harris; Barrett, Tom. “Energy in a Modern Society: XIV. Nuclear energy” (Course). Ohio State University.
Pratt, Thomas H. “Electrostatic Ignitions of Fires and Explosions” Wiley-AIChE Center for Chemical Process Safety.
