
Hello, curious thinkers and science enthusiasts! Have you ever stumbled upon a question so intriguing that it stopped you in your tracks? Here’s one for the books: Can water boil and freeze at the same time? It sounds like a riddle straight out of a science fiction novel, yet this phenomenon taps into the fascinating principles of physics that govern our natural world.
Have you ever seen water freezing and boiling at the same time? It is known as the triple point of water. When water is ice, liquid, and gas simultaneously, it becomes a triple point. The triple point combines water stages, in which water is a liquid state, solid and ice state, and vapor at gas coexist at a perfect temperature. This point is 0.01 degrees Celsius or 273.16 Kelvin. At this temperature, only you can see water at different stages simultaneously.
We’re going to dive deep into the realms of temperature, pressure, and states of matter to unravel this mind-bending query. So, strap in and get ready for a thought-provoking journey that will challenge what you know about water, its boiling point, and its freezing point. Let the exploration begin!
Water can boil and freeze at the same time
You must be thinking that water freezes at 0 degrees Celsius. Then how come it boils? The triple point can only exist in a vacuum or space—not that vacuum cleaner! We need to understand enthalpy vaporization in the vacuum chamber to understand how this occurs. This term says that boiling is not the main point of heating water.
There is another way to describe atmospheric pressure. The pressure caused by air is called atmospheric pressure. Atmospheric pressure changes with attitude. It can only happen in vast, large areas. Water boils at hundred degrees Celsius, but this is incomplete. It is defined as: “The boiling point of water is a hundred degrees Celsius at one atmospheric pressure.”
Now, what does this one atmospheric pressure mean? Water doesn’t always need to boil at a hundred degrees Celsius. You can boil it to whatever temperature you want. But you must also change the atmospheric pressure; for example, water can boil at 80 degrees Celsius at 6.9 Atmospherics.
- The atmospheric pressure is directly proportional to the temperature.
Let’s take the example of Earth. The air has atoms. The atoms also contain some weight. So the gravity will also cause on them. The air is mostly downwards near the surface than the Sky because of the weight of gravity. It means the atmospheric pressure will be more on the ground than in the Sky.
As the temperature is directly proportional to the atmospheric pressure, the ground pressure will be high, and the temperature will also be high. That’s why the Mountains are cold because they are located upwards.
How can water boil and freeze at the same time?
There is very little or no pressure in space or a vacuum. So if we put water in space or a vacuum, it will start boiling. There are two factors that determine whether the water boils.
Factor number one is When water boils, the water molecules in the liquid state become vapor. Then, the water molecules also heat the vapor molecules and gain energy. After some time, all the heat is gone with energy, and a vacuum sucks quickly. So this process is done very quickly. It is gone totally so that it will become ice.
As the heat molecules give their energy to their surroundings and make it equivalent, the surroundings also give their cold air to the boiling water and keep on boiling. The substance will go directly from solid to gas without very little pressure.
The second factor, air, also goes when the vapors go from the water to the vacuum. Then there is no air inside the water; water must compress, and heat forms ice.
There’s a time at 0.01 degrees Celsius when water is in all three stages. In thermodynamics, any pure substance can exist in three phases: a solid, a liquid, or a gas.
- Solid as Ice.
- Liquid as water state.
- Gas as vapor state.
That’s the time, which is known as the triple point. We can show these phases using a pressure-temperature diagram called a PT diagram.
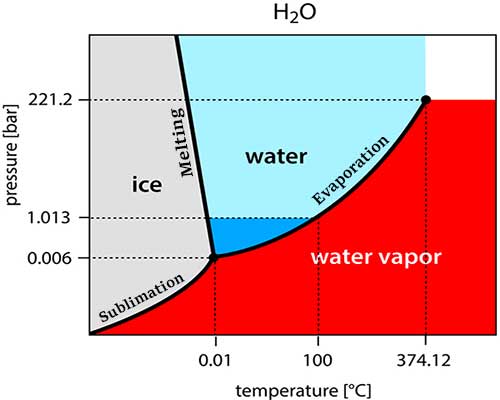
The graph shows the different phases of a pure substance with pressure and temperature.
- With pressure P on the vertical axis.
- The temperature is T on the horizontal axis.
The three phases are separated from each other by so-called separation lines. A melting line separates the solid and liquid phases, a sublimation line separates the solid and gaseous phases, and a vaporization line separates the liquid and gaseous phases. The triple point is the location where all of these lines are needed. Each substance’s specific pressure and temperature characteristics specify the triple point.
All three phases will be present simultaneously if a pure substance is like the specified temperature and pressure values. One says that the three phases coexist.
Pure water has a trigger point temperature of 0.01 degrees Celsius and a triple point pressure of 611.5 Pascal. This is the same as about 0.6% of the normal atmospheric pressure. At this point, water will exist both as ice and liquid.
Experiment
If you are curious about the triple point, you can make it yourself home. You need a vacuum pump, thermometer, pressure chamber, and magnesium sulfate anhydrous.
- First, you need to add magnesium sulfate anhydrous to the water. Magnesium sulfate makes the boiling process slow so that it does not harm the chamber.
- Now, put a thermometer in the water to measure the temperature.
- Put your beaker in the chamber and use the vacuum pump to remove all the air. Make the pressure low.
Now you can see the different states of water at a time!
This journey has not only shed light on a seemingly paradoxical question but also highlighted the endless wonders and complexities of the world around us. I hope this adventure has sparked your curiosity and encouraged you to ponder the mysteries that lie just beneath the surface of everyday phenomena.
Thank you for joining me on this fascinating voyage of discovery. Keep questioning, keep exploring, and always stay amazed by the incredible science that shapes our universe. Until next time, may your mind remain as open and boundless as the vast realms of physics!
More Articles:
Why is Space Temperature Cold?
References:
IUPAC, Compendium of Chemical Terminology, 2nd ed., “Triple point.”
James Thomson, “A quantitative investigation of certain relations between the gaseous, the liquid, and the solid states of water-substance.”

