A drug is a chemical or biological substance that affects the body and its functions. Drug absorption is when a drug enters the bloodstream. The absorption level can affect the drug’s speed and quantity at the site of action. It is termed bioavailability. If a tablet releases the drug quickly, blood levels may become too high, whereas slow release may result in low absorption levels.
Additional factors affecting absorption and bioavailability include the properties of the drug and the person’s physiology, such as the stomach’s pH levels and emptying speed. Therefore specific formulations are used to release the medicine at the desired speed. Standard formulations include tablets, capsules, suppositories, transdermal patches, and solutions. Drug metabolism is how the body breaks down drugs that mainly occur through enzymes in the liver.
Many factors include the intake of specific foods. Other drugs can increase or decrease the speed at which drugs are broken down and determine their blood levels. A drug may be broken down to stop it from working. However, other drugs must be broken down or modified before becoming active. These are known as prodrugs.
Some drugs are directly eliminated from the body by the kidneys in urine. For this to happen, the drug must be water-soluble. The liver or enzymes may metabolize drugs not water-soluble in the blood into breakdown products that can be eliminated in urine. Diseases that affect the liver or the kidneys can cause blood levels of a drug.
How does medicine work in the body?
Different medications target different biological processes and systems in the body to achieve their desired effects. Here are some common ways in which medicines work:
Binding to Receptors: Many medications work by binding to specific receptors on cells in the body. Receptors are proteins located on the cell surface or within the cell that recognize and respond to specific chemical signals. When a medication binds to a receptor, it can either stimulate or block the receptor’s activity, altering the cell’s response and influencing physiological processes.
Modulating Enzyme Activity: Enzymes are proteins that catalyze chemical reactions in the body. Some medications target specific enzymes, either inhibiting or enhancing their activity. Medications can affect biochemical pathways and processes by modulating enzyme functions, such as metabolism, neurotransmitter regulation, or hormone production.
Altering Neurotransmitter Levels: Neurotransmitters are chemical messengers that transmit signals between nerve cells. Medications that affect the central nervous system often work by modifying the levels or activity of neurotransmitters. They can enhance or inhibit neurotransmitter release, reuptake, or breakdown, influencing brain function and neuronal communication.
Interfering with Pathogens: Antibiotics and antiviral medications work by interfering with the growth or replication of pathogens such as bacteria or viruses. They may target specific structures or biochemical processes unique to the pathogens, preventing their proliferation and helping the body fight infections.
Modifying Gene Expression: Some medications, particularly those used in targeted cancer therapies, can influence cell gene expression. They may target specific genetic mutations or pathways associated with disease, helping to regulate abnormal cell growth and restore normal cellular functions.
The tongue is a thin mucous membrane rich in capillaries and blood supply. The medicine gets absorbed rapidly directly into the bloodstream. It means that it’s going to take effect more quickly. In oral medication, it will travel through the mouth, down the esophagus, and ultimately into the stomach, where stomach acids will start to break that pill down.
As that pill is broken down, it’s absorbed through the small intestine. But it passes through the liver before reaching other body parts where it can do the work. The primary benefit of taking solid pills is that most drugs are given orally. It’s because you can get a controlled dosage. In the end, it’s not about how fast it’s absorbed. It’s about the most effective delivery method for that specific medication.
A lot of medications work almost like a lock-and-key model. It’s looking for a specific receptor that it can fit into, and if it can’t fit that receptor appropriately, it does go right by it. So that’s where some of these new targeted therapies are.
Especially in the area of cancer, it’s looking for that particular receptor. It can get into the cell, and it might turn something on or turn something off. That’s going to help the body then fight the disease.
Drug/medicine absorption process in the body
Once drugs are inside your body, how do they move around and reach where they need to go? Do they stay in the body indefinitely, or are they eventually removed from the body somehow?
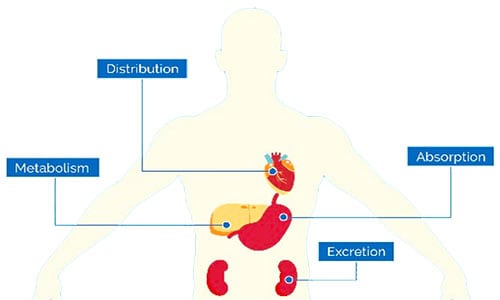
The study of pharmacokinetics deals with precisely these issues, and in learning about this, we can highlight four main processes. Those are absorption, distribution, metabolism, and excretion.
- Absorption generally describes the way the drug moves from its site of administration across one or more membranes, often into the
bloodstream if it was not administered there directly. - Distribution describes its journey through the bloodstream to target cells and specific target molecules.
- Metabolism describes how enzymes eventually get modified by enzymes and rendered ineffective.
- Excretion describes how it exits the body, typically through urine or feces.
We want to understand these processes and how interactions with food, drink, other drugs, and additional factors influence them. If a drug is administered topically, this could mean moving through the skin or a mucous membrane and subsequently through the walls of nearby blood vessels.
If administered orally, this would mean being absorbed through the lining of the stomach or intestines, a process in which the presence of food would slow. Some drugs will pass through the cells that comprise these membranes by passive transport, others by active transport. But one way or another, they will make it through on the way to their destination.
In describing this process, it will be appropriate to review the term “bioavailability.” This term describes the proportion of a drug successfully absorbed into the systemic circulation.
Next, distribution is how drugs move through the bloodstream after being absorbed or injected. Many factors, like how the drug interacts with blood components, such as plasma proteins, influence this. If the drug binds too tightly to these proteins, it will not reach its target cells.
Sometimes, a second drug is administered with the first with a higher affinity for these proteins than the first. Thus it serves the sole purpose of displacing the primary drug once bound to the protein. It allows it to be delivered to its destination. Beyond these blood elements, other factors may hinder the movement of a drug. These are anatomical barriers found in specific organs.
There are some blood-brain barriers. Some drugs will not surpass the barrier, while others will, such as psychotropic drugs or those affecting the mind. There is also the blood-placental barrier. That regulates which substances can pass from a pregnant woman’s bloodstream into the fetus. There are, however, many substances that can pass through this barrier. It can still harm the fetus, such as alcohol and certain medications.
There is also the blood-testicular barrier, which prevents many substances from reaching the male testes. Therefore it makes disorders of the testes challenging to treat. After distribution, there is metabolism. This describes any chemical reactions the drug may participate in, often aimed at inactivating and targeting it for excretion. A drug travels through the bloodstream and is highly likely to be metabolized.
For example, if a drug is taken orally, it will be absorbed through the intestinal wall. For this reason, the part of the circulatory system it enters is a collection of blood vessels called the hepatic portal system. These carry blood directly to the liver, which will be metabolized somehow. This is called the first-pass effect, referring to the first pass through the liver. It will typically significantly reduce the bioavailability of a drug.
In some instances, metabolism in the liver activates a drug, but this is less common. The first-pass effect can inactivate over 90 percent of an orally administered drug before reaching general circulation. It must be taken into account when determining the appropriate dosage. Drugs eventually get their target cells, but after enough time elapses, they will be metabolized.
Many different enzymes in the body perform these essential metabolic functions. The immune system is only good at dealing with large biological particles like viruses or bacteria. It has no defense against small molecules. So this detoxification mechanism explicitly aimed at small molecules had to evolve for life to exist in a chemical world.
Finally, after metabolization, the drug or its remnants exit the body, and there is excretion. This is typically done via exhalation, sweating, urination, or defecation. The kidneys are heavily involved in removing harmful substances from the bloodstream.
Some drugs are metabolized into gaseous form and are thus quickly exhaled. Some drugs are excreted through bile, a substance secreted by the liver to aid digestion. The bile is recirculated back to the liver via enterohepatic recirculation. Most of the drug can then be excreted by the kidneys, and the rest will exit as feces.
Glands produce fluids such as saliva and sweat, promoting excretion, though this method tends to be less effective. With that, we have traced the journey of a drug into the body, around the body, and out of the body. It gives us a basic understanding of pharmacokinetics.
How are new medicine developed?
The pharmaceutical industry is big business. According to a study published in the Annual British Journal of Clinical Pharmacology, pharmaceutical sales are approximately 365.6 billion dollars. A recent example of a company acquiring a life-saving drug and jacking up the price 4,000 percent overnight. But it’s not always about greed. One of the reasons for the high price of drugs is it’s so expensive to develop a new one.
Developing a new drug or medicine is a slow and expensive road. It can take decades and billions of dollars before a drug ever reaches the FDA, which has to approve it for use.
- According to The Association of the British Pharmaceutical Industry, about 25,000 chemical compounds were tested for every successful new drug. On average, 25 will have undergone clinical trials, and five will receive marketing approval.
According to a report published by the Tufts Center for the Study of Drug Development (CSDD), it costs more than $2.6 billion to make a drug that finally receives approval. So why does it take so much time and money?
The FDA, thankfully, has established strict guidelines for drug manufacturers and quality control measures like good laboratory practices and guidelines for clinical trials. With that in mind, drug discovery often starts with basic research.
Scientists might find mechanisms behind cellular receptors, ion channels, and enzymes. For example, research in the 60s on neurotransmitters led researchers to discover an imbalance of brain chemicals might have something to do with depression. Knowing that drug companies could get to work on the way to fix that imbalance.
Historically medicines were discovered in nature, like bark from the willow tree, which ancient cultures used to relieve pain. It turns out. The bark contained a compound that modern pharmaceutical companies use to make aspirin. Researchers can quickly predict what compounds will work using genetics and computer models and create many samples using specialized robots.
- According to a study published in the Pharmaceutical Journal, as many as 10,000 compounds may be considered and whittled down to 10 to 20.
Drugs must undergo rigorous testing to ensure they do what researchers suspect and don’t have overwhelmingly negative side effects. First, compounds must go through either phenotypic screens or target-based screens. Phenotype screening measures the test compound’s ability to affect cells, tissues, or whole organisms.
It’s pretty general. Target screening measures the effect of compounds on a purified target protein in a test tube, so it’s way more specific. It’s called an in-vitro assay. It targets the direct effect a molecule or compound has on the protein. Even with the best phenotype screening, the target screening is way more valuable to scientists and regulators.
Only compounds that show positive activity are developed further. They are made in larger quantities and subjected to more and more tests. Some compounds are tested on animals. Then they go on to clinical trials, which test the compounds in humans in tightly controlled, highly-regulated studies.
- Phase one trials test the safety of the new drugs.
- Phase two tests efficacy and phase three is the holy grail of science: double-blind, placebo-controlled studies.
After all of this, if the drug does what it’s supposed to do predictably, it can be submitted to the FDA for market approval. So you imagine with all this time, effort, money, and a huge failure rate. Pharmaceutical companies focus on drugs they think will have the best financial return.
More Articles
How Does Cold Medicine Work In Body?
Why Is Expiration Date Very Important?
Why Is Horseshoe Crab Blood Expensive?
How Do Enzymes Lower Activation Energy Work?
How Does pH Affect The Enzyme Activity?
References:
“Pharmacokinetic Pharmacogenomics.” Handbook of Pharmacogenomics and Stratified Medicine.
“Oral Drug Absorption.” Developing Solid Oral Dosage Forms. Elsevier.
“Drug Absorption – Clinical Pharmacology.” MSD Manual Professional Edition.
Kaplan Pharmacology 2010, page 6, Absorption.
