
Deodorant is a popular skincare product that helps keep underarms smelling fresh. Since it can not stop sweating, it is commonly used in winter to stop the odor from escaping pits. All human beings share one thing in common: stink. It’s a part of life, and each of us stinks uniquely.
The culprit is the human microbiota, whether stinky feet, bad breath, or rank underarms. The collective group of microorganisms lives on or in the human body. There are up to one million bacteria in underarms per square inch, making them odor factories. Sweat plays a significant role in odor production, but it isn’t the sweat that stinks. Instead, it’s the byproducts bacteria spit out when chomping on sweat. This is where deodorant comes in, killing or suppressing the bacteria by being mildly acidic to make it smell nicer.
How does deodorant work?
Deodorants work through various mechanisms to address the underlying causes of body odor. Here’s how deodorant typically works:
Odor Masking: Deodorants contain fragrances that help mask or cover up the natural odor produced by the body. These fragrances impart a pleasant scent that helps to mask any unpleasant body odor that may be present.
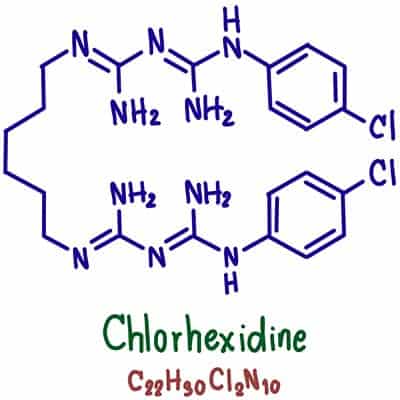
Antimicrobial Action: Many deodorants also contain antimicrobial agents that help inhibit the growth of odor-causing bacteria on the skin. These bacteria break down sweat and produce byproducts that contribute to body odor. Antimicrobial ingredients such as triclosan, chlorhexidine, or natural alternatives like tea tree oil can help reduce the number of bacteria, thereby minimizing odor.
Sweat Absorption: Some deodorants contain ingredients like talc or cornstarch that can absorb sweat. These ingredients help to keep the skin drier, reducing the conditions that bacteria thrive in and reducing the likelihood of body odor.
Antiperspirant Effect: Some deodorants also function as antiperspirants. These products contain aluminum-based compounds, such as aluminum chloride, aluminum chlorohydrate, or aluminum zirconium, temporarily blocking or narrowing the sweat ducts, reducing the amount of sweat that reaches the skin’s surface. Minimizing sweat production reduces the potential for bacterial growth and associated odor.
Modern deodorants don’t make it smell fractionally fresher. They also serve as antiperspirants, reducing the amount of sweat. The modern antiperspirant was invented in the 1940s by a man spectacularly French named Jules Montenier. All-natural deodorants cover the little buggers with essential oils, salts, or alum, but whether they work depends more on the person and their situation.
Two main glands under the arm produce sweat: the eccrine and apocrine glands. The primary suspect is the apocrine sweat gland, present in both armpits. Each of the pits contains up to 50,000 glands. The average human produces around a liter of sweat every day. Members of the Corynebacterium clan manufacture enzymes that break down sweat into various acids. Many include Propionic and Butyric acids, especially trans-3-methyl Hexeomic acid.
Eccrine lets out a water and salt solution to help cool down, while the apocrine gland lets out a mixture of fats and proteins. Bacteria then munch down on those apocrine secretions. It produces three primary odor compounds (3-methyl-2-hexenoic acid, 3-hydroxy-3-methyl hexanoic acid, and 3-methyl-3-sulfanylhexan-1-ol).
This one carries the weight of the typical human BO smell, but these two produce nice cumin and oniony addition to the mix. To stop this aromatic issue, deodorants apply certain compounds that kill or deactivate bacteria. While antiperspirants use substances that help block sweat glands, giving bacteria fewer nutrients to turn into gross smells. Even though the products differ, you can easily find hybrids at the store. Both always have some fragrance in them.
- Deodorant contains triclosan which is a compound that slows bacterial growth.
- Deodorant typically has a fragrance and alcohol. The alcoholic deodorant kills the pesky bacteria that are making the smell.
- The alcohol would evaporate, leaving behind Al-Cl-3, which, according to the American Chemical Society, would stop those eccrine glands, cutting sweat production.
Deodorants use chemicals such as triclosan to make the environment of the armpit too salty or acidic to sustain bacterial life. Triclosan has recently been under scrutiny due to its links to animal hormone regulation issues. Also, there may be links between triclosan and antibiotic-resistant bacteria. There’s evidence that they can reduce pheromones’ effect, the chemical markers that help us attract mates.
For this reason, the FDA is putting this stuff through a rigorous scientific review. But for the time being, triclosan is considered safe for human use. Many other antibacterial agents are used, and most deodorants also use alcohol, which sterilizes your armpit when applied.
Deodorant Vs Antiperspirant
Deodorants don’t stop sweating. They work by masking the smell because they have antimicrobials that kill the bacteria that cause it.
On the other hand, antiperspirants essentially block the darks that produce sweat by stopping sweating. Antiperspirants removed the bacteria’s food source, starving them into submission: no sweat or bacteria.
More Articles:
How Does Antiperspirant Work Chemically?
