
Antiperspirants have had aluminum salts as the key ingredient since the very beginning. Aluminum salts aggregate in pores, ultimately blocking sweat from getting through. The four most common versions (are Al Chloride, format, chloro-hydroxide, & zirconium tetrachlorohydroxide).
A type of sweat gland called the apocrine gland produces sweat with fats and proteins. Sweat contains lipids and amino acids that get broken down by bacteria. That process creates a distinctive body odor smell. Bacteria on the skin eat these fats and proteins and produce bad odors. The odor is gross. Deodorants make the environment too acidic or too salty for the bacteria to thrive, thus preventing the production of odors. On the other hand, antiperspirants enter the tube through which the sweat comes onto the skin.
How does antiperspirant work?
Antiperspirants work by reducing or temporarily stopping sweat production and minimizing body odor. They are commonly used to manage perspiration and control underarm wetness. Here’s a breakdown of how antiperspirants work:
Sweat Glands: The human body has two types of sweat glands: eccrine and apocrine. Eccrine glands are distributed throughout the body and produce sweat that primarily consists of water and electrolytes, regulating body temperature. Apocrine glands, found mainly in the armpits and groin area, produce a thicker sweat containing proteins and lipids that bacteria on the skin break down, resulting in body odor.
Active Ingredients: Antiperspirants contain active ingredients, most commonly aluminum salts such as aluminum chloride, aluminum chlorohydrate, or aluminum zirconium. These compounds work by temporarily blocking or narrowing the sweat ducts, reducing the flow of sweat to the skin’s surface.
Application and Absorption: Antiperspirants are typically applied to clean, dry skin in sticks, roll-ons, sprays, or gels. When applied, the active ingredients are absorbed into the sweat ducts, forming temporary plugs that obstruct the ducts and inhibit the release of sweat onto the skin.
Sweat Reduction: By blocking the sweat ducts, antiperspirants reduce the amount of sweat that reaches the skin’s surface. This helps to minimize underarm wetness and can provide a dry sensation, especially in situations where perspiration is a concern, such as during physical activity or in hot weather.
Odor Control: Besides reducing sweat, antiperspirants also contribute to odor control. By reducing the amount of sweat available on the skin surface, there is less moisture for bacteria to break down, resulting in less body odor.
Antiperspirant prevents sweat production and bacterial growth, invented in 1903. It creates a partial vacuum around the armpit, which absorbs all things in the general vicinity, mainly smells.
Antiperspirants usually have fragrances and alcohol. In addition to those ingredients, they contain an aluminum compound, usually aluminum chlorohydrate, which reduces sweat. These clog those sweat glands able to prevent sweating.
Both deodorants and antiperspirants often use cyclomaticones. Cyclomethicone is fast drying silicon compounds as solvents. So the chemical formula of it is C10H3O5Si5.
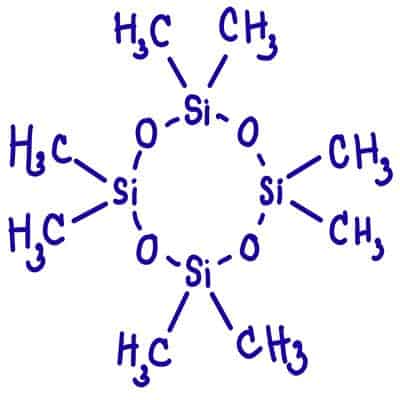
Every hydrogen atom is bonded to a carbon atom. Every single carbon atom has three hydrogen atom bonds and one bond to a silicon atom. Their atomic force is key to stopping sweating and bacterial growth.
There are two types of sweat glands all over the skin: Eccrine and Apocrine.
First, there are most eccrine glands, and they kick in to cool. Heat, physical exertion, stress, and nervousness can stimulate them. Eccrine glands excrete water and salt and, for the record, have nothing to do with body odor.
On the other hand, apocrine glands carry secretions of fat and proteins along with your sweat. Once this hits the exterior surface of the skin, those fats and proteins are a buffet for bacteria that excrete.
Working process
Antiperspirants have an active ingredient that gives them their sweat-blocking powers, usually an aluminum-based compound like aluminum chloride or aluminum chlorohydrate. When this stuff enters the duct leading into an eccrine gland, aluminum ions enter the cells lining the duct walls.
Water molecules pass into the cells with them. The cells around the duct begin to swell with the water, enough to squeeze the ducts closed. Sweat can’t get out then, and you’re stain-free for a while. But each cell can only draw in a certain amount of water. So eventually, the water concentrations outside and inside the cells reach equilibrium.
When this happens, water inside the cell begins to pass back out of the cell through osmosis. The swelling goes down, reopening the duct. That is why people have to reapply for antiperspirants.
Also, it acts astringently to cause pores to contract. More importantly, antiperspirants are also trying to help regulate body temperature. Research suggests that even extreme body odor can be reduced through diet changes and exercise.
More Articles:
