
Glass has a strange structure since it’s made by melting and cooling silicon dioxide. Silicon dioxide forms when silicon and oxygen react in the Earth’s crust. As they react, the molecules arrange themselves in a regular crystal known as quartz.
However, it loses this crystal structure when it is melted as it cools, and the molecules lose energy. They’re not able to move back into the orbit of the crystal. So instead, form a random structure like that of a liquid. This liquid light structure is called an amorphous solid. It gives the glass a uniform surface meaning and does not scatter the lime in different directions.
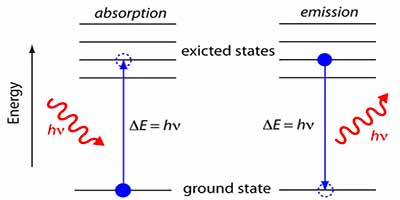
The electrons around the nucleus are at specific energy levels in an atom. The lowest energy shelf is called the ground state. Electrons can move up to a higher shelf if they have enough energy. Otherwise, their energy is too small and can’t quite reach the shelf.
If photons of light have the same energy difference between the two energy shells, the electron can absorb the photon and gain energy. It gives the electron a leg up onto the higher shelf. Unfortunately no! If the photon’s energy is not the same as this difference, it will pass straight through, and the electron can’t absorb it and so can’t move up. This is what happens in the glass.
What is glass?
Glass is primarily made of silica dioxide, a white powdery substance. It is also the main constituent of sand. It can be added to sodium carbonate, lime, oxide, and iron. However, most things are added to lower the melting point or change other properties within the glass.
Glass is technically any rigid amorphous solid, meaning its atoms and molecules aren’t arranged in an orderly structure. But rather, in whatever random arrangement they happened to be in when the material cooled and solidified. It’s as though a liquid stopped moving all of a sudden. Unlike ice, the water molecules tug on each other and lock themselves into a repeating crystal pattern. As the glass cools, its molecules contract until they stop moving altogether.
How is glass made?
Human bodies aren’t transparent to visible light. Visible light and X-rays are different forms of electromagnetic radiation with different wavelengths and energies. So what’s the difference? Glass is transparent to visible light. It is made up of a bunch of silicon and oxygen atoms. Same as this stuff, sand!
By melting sands into a liquid and cooling them down fast, they froze in place in an organized jumble. Wave, quantum-y electron clouds surround all those atoms. But the electrons around a nucleus can’t be anywhere. They live on specific energy levels. When a photon comes by with the right amount of energy, it gets absorbed, bumping an electron to a higher energy level. But if that photon doesn’t have the right energy, it passes by for the particular atoms that make up glass.
Structure of glass
Inside the earth’s crust, silicon and oxygen react together to form silicon dioxide, and the molecules of this mixture come together to create a common crystalline form called quartz. It is the main ingredient of most types of glass.
When we burn the quartz at a high temperature, the heat energy puts pressure on the molecular chain of silicon dioxide. It breaks them apart and converts them into flowing liquid. When this liquid silicon dioxide cools down again, it does not transform into a solid crystal.
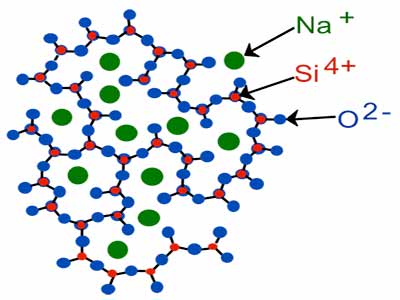
That’s because as the molecules lose energy, they cannot move into an ordered position and form a chain as they used to before. Also, it converts into something called an amorphous solid. A material with a disordered liquid composition allows the molecules to fill any gaps. It makes the surface of the glass uniform, allowing light to pass through it without getting scattered in different directions.
Why is glass transparent?
The energy levels are so far apart that no visible light photons have enough energy to move the electrons up. So they all pass through unabsorbed. Shortly, It is the reason for transparency.
- Electrons do not have size. They can only occupy specific energy levels. If energy is definite, the position is not.
If electrons are simultaneously everywhere, the photon will hit one no matter what, and electrons absorb the photon. They can only absorb certain types of light. Jumping up energy levels is not the only way electrons can absorb light. There would be no such thing as reflection or refraction if it were.
There are so many photons hitting so many electrons that photons go everywhere inside the glass. Those photons interfere and cancel each other in places that don’t cancel the light. Traveling in a new direction is determined by how the atoms are organized. When atoms bond to form solids, the energy levels move closer to forming bands. This leaves large energy gaps between them.
- The gap between bands in glass is about 9 electron volts, too big for visible light. To excite electrons, the photon has to have an energy of at least 9 electron volts. It means its wavelength must be at most 138 nanometers. That’s ultraviolet light.
It won’t absorb thermal energy if visible light can’t excite electrons. It still gains absorbed as the vibration and the electrons. Since the electrons always reject those photons, they make it out of transparency.
The energy levels are so far apart that visible light doesn’t have enough energy to boost the electrons to the next level. That’s why visible light passes right through! But photons of UV light have the right amount of energy to power up those electrons. They get absorbed. This is why glass is opaque to most UV! Why it’s hard to get a sunburn through a window.
Visible light has a higher frequency, so it must have higher energy photons than visible light. This results from the black Einstein equation (E = hf). So maybe one of them has not too much energy but not too little. Their energy is right when it turns out, for a glance, the Goldilocks region of energies lies in the ultraviolet. It means the electrons absorb the ultraviolet photons and don’t pass through.
Being transparent depends on the relationship between light energy and an atom’s electrons. Different elements have different energy requirements for their electrons to absorb light. When visible light hits atoms, it’s absorbed. Some light might get through a few top layers of skin cells, but within a few millimeters, all the photons get gobbled up. That’s why everything is not transparent.
Frequently asked questions
How does the light pass through the glass?
To find the answer, we need to dig deeper into the glass to observe the atoms of silicon dioxide. Atoms are made of a nucleus in the center surrounded by electrons orbiting around them. But an atom is mostly empty, and there is plenty of gap between the orbits of electrons. This empty space leaves much room for the particles of light called photons. It passes through the atomic structure of glass without getting absorbed by electrons, and the glass looks transparent.
Why do other objects look opaque and not transparent?
That’s because the gaps between the electrons of an opaque object are tiny. These tiny gaps do not allow the photons to pass through it and absorb electrons, making the object look opaque. High-temperature glass can naturally form when sand is struck by lightning. Also, another amazing fact about glasses is that when it breaks, the cracks can move at 3,000 miles per hour, five times faster than the average airplane, which travels at 575 miles per hour.
Does light slow down in glass?
Dividing the distance by time gives us a speed less than the speed of light. It gives you an average speed. Its atoms vibrate when the light beam comes into contact with the glass. That vibration causes all those atoms to emit their light. Most light cancels except for one particular direction: the forward direction. When combining all those waves, they look like one beam traveling slower than the speed of light. But is the light traveling slower?
That depends on what you mean by “light.” It’s a language issue. One single photon is “light,” but this beam has a bunch of photons in it. If you also refer to that beam as “light,” then, sure, “light” slows down in materials. But, It’s physically impossible for light not to go at the speed of light. Beams of light don’t always have the same properties as their photons.
Is glass a solid?
Glass isn’t technically solid or liquid. It’s an amorphous solid lacking other solids’ crystalline or polycrystalline structures. This lack of a pattern and weak chemical bonds explains how glass can behave like a brittle solid. Also, it accounts for the glass-liquid transition, which is when heated glass is between a supercooled liquid state and a solid state. It behaves like a rubbery viscous liquid.
The molecules on the glass surface never stop moving, even when cooled. They do move extremely slowly, but they move nonetheless. A common myth about glass is this movement. Antique glass has sagged over the years. Many old buildings and church windows have thicker glass on the bottom.
That’s likely an imperfection in how the glass was made because a flat pane of glass to sag at room temperature would take longer than the universe’s age. Plus, even older glass from Egypt doesn’t have that sagged quality, so that myth goes. The lack of a crystalline structure is often used to explain glass transparency.
Why does light change direction in glass?
Light traveling through the air will change its direction when it hits the surface. The amount that its direction will change depends on the angle at which it hits the surface. It changes the incident angle, and the transmitted angle will change correspondingly. Those angles are related through a physics formula called Snell’s Law, named after Dutch astronomer Willebrord Snellius, who lived in the 1600s.
Three commonly suggested explanations are wrong or incomplete: Fermat’s Principle, the analogy of soldiers marching, and Huygens’s Principle. Fermat’s principle says that light will travel from one point to another, taking the minimum time. Light follows the path of the shortest time.
After Dutch physicist Christian Huygens, Huygens’s principle depends crucially on the wave nature of light. This principle is called diffraction, and it’s universal to all waves. If waves hit more than one gap, each gap will allow waves to pass through and make ringlets. The wave’s gaps can interfere with one another, either adding to or subtracting.
When two waves’ peaks encounter one another, the result is a single and bigger wave. When a peak of one wave hits the trough of another wave, they can cancel each other out entirely. What Huygens claimed is that there is no need for gaps. Each spot on a wave acted like it went through a gap and spread out.
Light enters glass or water or any transparent medium, and it bends. The reason that it bends is that the epsilon in glass is bigger than in air. It’s there because of how the electric fields from the light interact with matter in the glass. The glass has charged in it, but they’re arranged randomly. The electric field makes the charges move around, which sets up a counterbalancing electric field from the charges.
The result is that the electric field in the glass is lower than in the air. It is because the electric field from the glass is in the opposite direction. This is why the perpendicular electric field is lower in the glass.
More Articles:
Why Do Some Nerds Wear Glasses?
What Causes The Optical Illusions?
References:
Zallen, R, The Physics of Amorphous Solids. New York: John Wiley. pp. 1–32. ISBN 978-0-471-01968-8.
Cusack, N.E., The physics of structurally disordered matter: an introduction. Adam Hilger in association with the University of Sussex press. p. 13. ISBN 978-0-85274-829-9.
Scholze, Horst, Glass – Nature, Structure, and Properties. Springer. pp. 3–5. ISBN 978-0-387-97396-8.
Elliot, S.R., Physics of Amorphous Materials. Longman group ltd. pp. 1–52. ISBN 0-582-44636-8.
Neumann, Florin. “Glass: Liquid or Solid – Science vs. an Urban Legend.”
Gibbs, Philip. “Is glass liquid or solid?”.
Table of Contents

