Our kidneys eliminate more water as urine than we take in as seawater. As adults, about 5 liters of blood flow in our bodies. It plays a vital role in helping to deliver oxygen and other nutrients and serves as a highway for our immune systems.
Also, it helps control the concentration of electrolytes bathing every cell in our bodies. But why does seawater make our body dehydrate or cause water retention? To know the answer, stay with me.
Why does salt water dehydrate you?
Saltwater can dehydrate the body because of its high salt concentration. When we consume salt water, the body recognizes the higher salt concentration and tries to balance it by removing the excess salt through urine. However, to do so, the body requires more water.
Here’s how saltwater dehydration occurs:
Osmosis: Our body maintains a delicate balance of fluids inside and outside our cells. Osmosis is water movement across cell membranes from an area of lower solute concentration (lower salt concentration) to an area of higher solute concentration (higher salt concentration). In saltwater, which has a higher salt concentration than our body fluids, water moves out of our cells through osmosis.
Increased Urination: When we consume saltwater, our kidneys work harder to filter out the excess salt. The body requires additional water drawn from our cells and body fluids to eliminate this salt. This leads to an increased frequency of urination, resulting in a net water loss from our bodies.
Dehydration: The body becomes dehydrated as water is continuously drawn out of our cells and body fluids due to osmosis. This imbalance can lead to increased thirst, dry mouth, fatigue, dizziness, and more severe complications if not addressed.
Water dissolves stuff, especially soluble compounds that hold a charge like most metals or polar ones. These can all be found in our blood and must be held at an appropriate concentration for our cells to function.
One of the most important to consider because of its sheer abundance is sodium, which is salt combined with chloride.
- If sodium levels rise in the blood, water rushes out of cells via osmosis and into the blood to lower its sodium concentration, leaving behind shrunken, parched cells.
- If sodium levels drop in the blood, water rushes out from the blood to increase its sodium concentration and into cells, causing them to swell up.
Our kidneys help regulate sodium concentration. In some regards, they care more about sodium than water itself. If you eat salty food, your kidneys have two primary responses: Send signals to make you thirsty in the hope that you drink water to account for this extra sodium, and concentrate sodium into urine to eliminate some excesses.
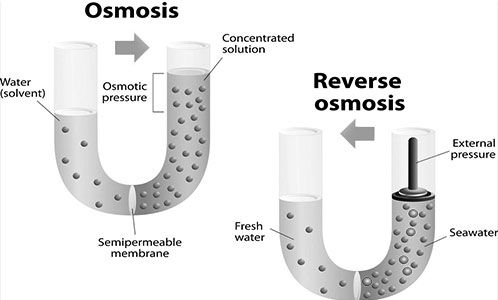
With seawater, our kidney’s regulatory mechanisms aren’t enough. They make us thirsty, but we don’t have any salt-free water to drink. They concentrate sodium into the urine but can’t concentrate it enough. Some estimates put the maximum sodium concentration in our urine at about half that of seawater.
So to get rid of all that extra sodium, with less sodium per unit volume in urine than in seawater, they have to get rid of more water than we did. To regulate sodium concentration, our kidneys dehydrate us even further.
How does salt water dehydrate you?
We have osmoreceptors in the hypothalamus in the brain. That is responsible for sensing sodium and water balance within our bodies. When sodium is consumed, our bodies release water to escort the sodium out in our urine. Then we are left with less water.
So the same Osmoreceptors detect this state and trigger a sensation of thirst to get you to drink more water, to replenish your water stores so your body can return to the proper concentrations of electrolytes.
It is a big reason why places like bars will offer salty snacks. The salt causes us to become thirsty and then order more alcoholic drinks. So water tension may occur because the pressure inside our capillaries changes. Capillaries are tiny blood vessels that carry interstitial fluid composed of nutrients, oxygen, etc.
Our capillaries carry this fluid into our surrounding tissues. When you overeat salt, it increases sodium levels in your body. So your body will hold onto it and retain more water to dilute the salt.
The retention of this fluid within our tiny capillaries causes pressure, and then they leak out this excess water into our surrounding tissues. When this fluid gets into the tissue space between our cells, this causes edema, water retention, or dehydration.
- The more fluid released, the more and more of it will remain in our skin tissue rather than returning to our capillaries.
So this results in swelling and waterlogging. The water moving from our bloodstream to our skin tissue when we consume salts causes that puffy look.
Does swimming in salt water dehydrate you?
Swimming in salt water does not typically cause dehydration. The saltwater itself does not dehydrate the body. When you swim in salt water, your skin absorbs some of the water, but the amount is generally minimal compared to the overall water content in your body.
However, it’s important to note that spending time in saltwater, especially in the sun and under hot conditions, can contribute to dehydration indirectly. Here’s why:
Sweating and Evaporation: While swimming in saltwater, your body still produces sweat to regulate body temperature. As the sweat evaporates from your skin, it can contribute to fluid loss and potentially increase the risk of dehydration if you don’t replenish the lost fluids.
Reduced Sensation of Thirst: When immersed in water, whether it’s saltwater or freshwater, you may not feel as thirsty as you would when you’re on dry land. This can lead to a reduced sensation of thirst and a decreased tendency to drink water, which may contribute to dehydration if you’re not mindful of your fluid intake.
To prevent dehydration while swimming in saltwater, it’s important to:
- Drink fluids before, during, and after swimming to stay adequately hydrated.
- Pay attention to your body’s signals and drink water even if you don’t feel particularly thirsty.
- Take regular breaks from swimming and rest in the shade to avoid overheating.
- Be mindful of environmental conditions, such as high temperatures and sun exposure, which can increase the risk of dehydration.
- By staying hydrated and being aware of your body’s needs, you can enjoy swimming in saltwater without significant dehydration concerns.
What are the benefits of drinking seawater?
Sodium is an essential nutrient for humans. It regulates blood volume, pressure, body fluids, and osmotic equilibrium. It is also necessary for many metabolic processes, nerve transmission, heart activity, circulatory system, and muscles.
Sodium is so essential to our diet that we have retained the salty taste buds on our tongues for hundreds of thousands of years. So the body has a complex system for maintaining an optimal sodium balance. You need a minimum of 180 milligrams of sodium per day. Consuming more than 2300 milligrams is considered unsafe, according to the Centers for Disease Control and Prevention.
Yet the average American gets over 3400 milligrams of sodium a day. So your body tries to maintain a sodium water concentration outside your cell walls, similar to seawater. When you consume sodium, your body holds onto the water to keep that ideal concentration. When you consume 400 milligrams of sodium, your body will compensate by retaining four cups of water within your body.
A high sodium diet can cause more issues than only water weight gain. It also elevates your blood pressure because more water is in your blood trying to dilute the salt, and therefore, there’s more pressure in your vessels and arteries. It increases your risk of having a heart attack or stroke. So sodium is highly tied to water within our bodies.
How do you add salt to food?
Our minimum sodium requirement is 180 milligrams a day, and we will reach this minimum on a whole-food, plant-based diet. Plant foods contain sufficient macro or micronutrients, whether protein, carbohydrates, fats, vitamins, or minerals.
They are available in much better concentration plant sources than you’d find in animal products. If you still want to use additional salt on your starch meals, you can do this but don’t add salt when preparing your meal.
So when you’re cooking your soup, don’t add any. When it’s in your bowl, sprinkle a little salt on the surface, and then those salt granules will hit your taste buds directly. Also, you’ll get that satisfaction from the salt without taking in a lot of sodium.
Read more similar topics:
How Long Can You Survive Drinking Sea Water?
What Are The Reasons For Sea Water Salty?
What Makes Salt To Make Food Taste Better?
Why Can Not We Use Ocean Water To Put Out Fires?
References:
Mange K, Matsuura. “Language guiding therapy: the case of dehydration versus volume depletion.”
Ashcroft F, Life Without Water in Life at the Extremes. Berkeley and Los Angeles.
Riebl SK, Davy BM. “The Hydration Equation: Update on Water Balance and Cognitive Performance.”
Table of Contents
